Intravitreal injection is one of the most popular, but at the same time, the most effective methods of treating ophthalmological diseases. The first intravitreal injection was performed by Ohm J. in 1911 for the purpose of tamponade of the retina with sterile air. In the 40s of the 20th century, interest in this procedure began to grow, and experimental studies were carried out on the intravitreal use of antibacterial drugs in the treatment of inflammatory eye diseases. In the 1970s, work was carried out on the treatment of proliferative vitreoretinopathy using intravitreal injection of antioneoplastic drugs. In the 1980s and 1990s, intravitreal administration of antiviral agents was explored.
In the last decade, intravitreal administration of an angiogenesis inhibitor has become widespread, which is a highly effective method of treating retinal diseases accompanied by the growth of newly formed pathological vessels.
Thus, with the development of scientific ideas about the pathogenesis of eye diseases and the improvement of technologies in the pharmacological industry, intravitreal administration of the drug has become an option in the treatment of various pathologies of the organ of vision, and the list of drugs used has expanded significantly.
What is intravitreal drug administration?
The inside of the eyeball is filled with a clear, gel-like substance called the vitreous humor. Intravitreal injection involves the introduction of a drug into the cavity of the eyeball using a needle. Most medications, when used in the form of eye drops or ointments, do not reach the retina. While the bulk of ophthalmic pathology requires drug delivery directly to the retinal area.
The required effect can only be achieved through intravitreal administration of the drug, since the presence of a special cellular barrier does not allow medications injected into a vein or muscle to reach the retina. This treatment method plays a vital role in the treatment of many eye diseases. Each disease requires the administration of certain medications, which will be discussed below.
The list of drugs and indications for their administration continue to constantly expand, and scientific research in this direction is being carried out all over the world at the present time.
Indications for intravitreal injection
The list of indications for intravitreal administration of drugs is determined individually depending on the eye disease present in each individual patient. Using this method, you can successfully treat severe fundus diseases that potentially lead to complete or partial loss of vision, such as:
- Senile macular degeneration, wet form.
- Clinically significant macular edema resulting from proliferative diabetic retinopathy.
- Endophthalmitis.
- Uveitis.
- Occlusion of the central retinal vein or its branches.
- Choroidal neovascular membrane secondary to multiple retinal pathologies.
- Cystic macular edema.
Advantages and disadvantages of the method
Like any therapeutic method, intravitreal administration of drugs has its pros and cons. The undeniable advantage is targeted delivery, a high probability of a full therapeutic effect and a relatively low risk of systemic adverse reactions. These advantages and the resulting therapeutic effect are very tempting for ophthalmologists. However, the risks of possible complications should always be on the other side of the scale.
The most serious complication of the procedure is bacterial contamination and endophthalmitis resulting from the injection. Recent scientific research in this area estimates the risk of endophthalmitis to be 0.051% for any given injection. However, if the medical staff observes all aseptic measures, the risks of bacterial contamination are minimized.
Other documented complications include:
- Retinal detachment.
- Persistent intraocular hypertension.
- Subconjunctival or intraocular hemorrhages.
- Damage to the lens.
- Development or progression of an existing cataract.
- Hypotension, allergic or anaphylactic reactions.
When planning intravitreal drug administration, the patient must be explained in detail all the advantages of the method, as well as the risks and possible complications. The procedure begins only after obtaining the patient's voluntary informed consent.
Technique of the procedure
Intravitreal injections are performed on an outpatient basis, that is, after the procedure the patient does not need hospitalization and can go home after short-term observation and receiving medical prescriptions. Due to the small diameter of the needle used, its insertion into the eye cavity is accompanied by minimal discomfort, as a result of which the use of anesthetic eye drops is usually quite sufficient for the patient’s surgical comfort. In rare cases, a subconjunctival injection of lidocaine is used for additional pain relief. To prevent bacterial contamination, the conjunctival sac is treated with an iodine-containing antiseptic. Only sterile surgical instruments are used, and the doctor performs thorough hand cleaning and uses sterile gloves. Intravitreal administration of an angiogenesis inhibitor, steroid drugs or antibiotics is carried out according to standard techniques.
An eyelid speculum is used as a retractor. It is necessary to prevent involuntary blinking of the eye, which can interfere with the doctor’s work. Preoperative administration of an antibiotic remains at the discretion of the attending physician and is more often used in the presence of concomitant infectious conditions, in particular blepharitis. However, scientific studies have not obtained reliable data on reducing the risk of endophthalmitis with the preoperative use of antibacterial drugs. For intravitreal administration of drugs, needles with a thickness of 27-30G are used. The injection site should be 3.5-4 mm from the limbus for people with their own lens. For patients after lens replacement, the distance should be 3-3.5 mm. These distances are based on the anatomical features of the eyeball: the needle must pass in a special place, called pars plana in Latin, bypassing the lens and retina. During the injection process, the patient is asked to look in the opposite direction from the quadrant in which the intervention is being performed. After the needle enters the vitreous cavity, the drug is slowly administered.
After performing the manipulation, the surgeon must ensure that there is adequate circulation through the retinal artery. Sometimes postoperative ophthalmoscopy is necessary to ensure that there are no complications. Postoperative antibiotic prophylaxis also remains at the discretion of the ophthalmologist. Primary monitoring of intraocular pressure is carried out immediately after the injection, then after a 5-10-minute interval. If no decrease is observed after 20 minutes, the use of antihypertensive drugs is recommended. Once all precautions have been taken, the patient may leave the ophthalmology clinic.
Discussion
Summary of the main finding of the study
The results obtained indicate the effect of IV ranibizumab on the content of the key RAS enzyme, ACE, both in the systemic circulation and locally in the SF.
Before treatment, patients with DME showed an almost twofold increase in the content of ACE in the SF, with a simultaneous twofold decrease in the concentration of this enzyme in the SF. Under the influence of antiangiogenic therapy, there was a decrease in ACE in S.Zh. Moreover, this pattern was noted both for the eye with DME and IVV, and for the fellow eye where the injection was not performed. The level of ACE in the SC of patients with DME gradually increased after injection, but did not reach the levels of the control group.
In patients with AMD, before treatment, the concentration of ACE in the SC and SF was practically no different from the control values. After IV ranibizumab, there was a decrease in the initially normal levels of ACE in S.Zh. Moreover, this was noted both in the eye with vAMD, into which the injection was performed, and in the fellow eye. The concentration of ACE in the SC did not change significantly against the background of IVV in AMD.
Discussion of the main result of the study
The change in ACE concentration in the SF in the fellow eye, which did not undergo IVV, is an interesting observation. One could think about a systemic effect of the drug, but the absence of changes in the concentration of ACE in the SC contradicts this assumption and suggests oculo-ocular interactions caused by a complex set of neurohumoral mechanisms [14].
RAS plays an important role in the progression of DR [15]. An increase in the concentration of AII, ACE, and VEGF in eye tissues increases the risk of neovascularization and increased vascular permeability [16]. An increase in the concentration of AII, VEGF and prorenin in the vitreous body in patients with severe PDR has been proven [17].
Previously, when studying ACE activity in patients with DR, we found its increase in the SC as the disease progresses, with a simultaneous decrease in ACE activity in the SG [18]. In this study, on the contrary, an increase in the concentration of ACE in the SF of patients with DME was found. Perhaps these differences are associated with the action of endogenous enzyme inhibitors [19]. The data obtained indicate the need to compare changes in systemic and local ACE content and activity in DR.
It is known that ACE is involved not only in the formation of AII, but also in the breakdown of bradykinin, which, with a decrease in ACE content, can accumulate in eye tissues [20]. Bradykinin belongs to the kallikrein-kinin system and promotes vascular permeability, causes vasodilation and can increase tissue edema. The decrease in ACE concentration in the SC of patients with DME that we discovered may be the reason for the lower supply of this enzyme with the bloodstream into the retina and lead to the accumulation of bradykinin, creating conditions for the appearance of macular edema. Reducing the amount of bradykinin could reduce the risk of DME, which opens new prospects for the treatment of this condition. It is possible that a decrease in ACE levels in the blood is a characteristic feature of patients with DME and can be used as an additional prognostic criterion for edema in patients with DR.
A decrease in the concentration of ACE in the SF in patients with DME and AMD after IV ranibizumab indicates the relationship between the local RAS and the angiogenic system and justifies the search for new possibilities for the treatment of DME by correcting the RAS.
Intravitreal administration of Lucentis
Lucentis is an angiogenesis inhibitor whose active ingredient is ranibizumab. The mechanism of action of Lucentis when administered intravitreally is due to the blockade of receptors necessary for adhesion and binding of endothelial vascular growth factor. Ranibizumab interacts with receptors on the surface of endothelial cells to prevent cell proliferation, fluid leakage, and the formation of abnormal blood vessels.
Lucentis is widely used in the antiangiogenic therapy of neovascular form of senile retinal degeneration, macular edema associated with retinal vein occlusion, diabetic retinopathy and macular edema that occurs against its background. The most commonly used dosage is 0.5 mg of Lucentis once a month. The duration of treatment is determined by the attending physician, usually it is about 6 months.
Intravitreal administration of Avastin
This drug also belongs to the group of antivasoproliferative agents, the active ingredient of which is bevacizumab. Previously, monoclonal antibodies of this kind were used to treat oncological pathologies, in particular, malignant tumors of the stomach and intestines. Later, the effectiveness of bevacizumab was proven when administered intravitreally as an angiogenesis inhibitor in the treatment of diabetic retinopathy and wet retinal dystrophy.
The mechanism of action is similar to that of ranibizumab (Lucentis). Avastin allows you to stop the formation of new blood vessels and the leakage of fluid through the vascular wall, which helps patients maintain visual function for as long as possible.
Intravitreal injections
In terms of complexity and technical details, intravitreal administration of Eylea solution is very close to microsurgical operation. In this regard, it is performed by an experienced surgeon in a sterile operating room.
In preparation for the injection, nearby skin is treated with a disinfectant solution. Epibulbar (drip) anesthesia is administered, which completely blocks the sensitivity of the scleral surface.
https://www.youtube.com/watch?v=https:accounts.google.comServiceLogin
The injection is performed with a special syringe, which is used to partially puncture a certain area of the ciliary body to a depth of 3 mm. The drug is injected by slowly pressing the piston, after which the needle is removed. No puncture fixation or sutures are required.
At the end of the procedure, monitoring of intraocular pressure is mandatory. If it increases significantly, paracentesis can be performed - a puncture of the cornea in the limbal region.
At the final stage, special drops with antimicrobial and anti-inflammatory effects are instilled into the eye, which must continue to be dripped according to a certain pattern for the next three days at home.
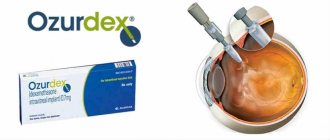
Intravitreal administration of Ozurdex
Ozurdex is a drug for intravitreal injection containing 0.7 mg of the steroid hormone dexamethasone. The purpose of intravitreal administration of Ozurdex is to suppress the inflammatory response by suppressing a variety of inflammatory cytokines. Clinically, this manifests itself in a decrease in edema, fibrin deposition, minimization of capillary sweating and migration of inflammatory cells.
Indications for intravitreal use of Ozurdex are occlusion of the retinal vein (main trunk or branches), uveitis of the posterior segment of the eye and diabetic macular edema. The issue of repeated injection of Ozurdex is decided after assessing the dynamics of the condition of the fundus. The administered drug has a prolonged effect.
What side effects may occur with anti-VEGF drugs and intravitreal injections?
After an intravitreal injection, mild pain, redness of the eye (bleeding at the injection site), burning and lacrimation (especially after the effect of anesthesia) has worn off. Increased sensitivity to light, foreign body sensation, and floaters are also possible. These side effects are temporary, do not require any treatment or intervention, and resolve on their own within a few hours or days. Serious complications with intravitreal injection are extremely rare.
Intravitreal administration of Gemaza
The chemical substance contained in this drug is prourokinase. It belongs to fibrinolytic agents, that is, it has the ability to transform plasminogen into plasmin, thereby lysing already formed blood clots. For intravitreal administration, 500 IU of the active substance is used.
Indications for use: partial hemophthalmos or subtotal and total hemophthalmos without signs of retinal detachment according to ultrasound examination, occlusion of the central vein or retinal artery or their branches, sub-, intra- and preretinal hemorrhages. When used locally, the risks of systemic bleeding are minimal or absent. The drug is contraindicated in cases of blood diseases associated with hypocoagulation, as well as in cases of severe liver failure.
Intravitreal injection of Kenalog
The active ingredient in Kenalog is triamcinolone. This is a synthetic glucocorticosteroid hormone. Its effect is based on the suppression of angiogenesis and inflammatory reactions by blocking the migration and proliferation of inflammatory cells. Steroids also block the production of endothelial growth factor, stabilize cell membranes and reduce vascular permeability, which is important for the treatment of a wide range of ophthalmic diseases.
Intravitreal administration of Kenalog is indicated for diabetic and cystoid macular edema, retinal vein occlusion, as well as the wet form of age-related retinal degeneration.
Intravitreal antibiotic injection
Intravitreal administration of antibacterial drugs is the most effective way to treat endophthalmitis. Intravitreal administration of antibiotics is the only way to achieve their therapeutic concentration in the vitreous cavity, since the blood-retinal barrier does not allow drugs from the systemic circulation to penetrate into the cavity of the eyeball. With timely use, it is possible to obtain a good therapeutic effect and maintain high visual functions.
To treat bacterial endophthalmitis caused by gram-positive flora, drugs such as Vancomycin and Cefazolin are used. For gram-negative pathogens, the use of Ceftazidime, Amikacin and Gentamicin is recommended. Sometimes antibacterial drugs are combined with intravitreal corticosteroids.
Intravitreal administration of Eylea
Eylea is also an angiogenesis inhibitor whose active ingredient is aflibercept. Each syringe of Eylea solution contains 40 mg of active substance. Aflibercept is a monoclonal antibody with pronounced antiproliferative properties.
The mechanism of action is to bind and prevent the activation of endothelial growth factor and placental growth factor. Thus, the effect of the drug is also to block neovascularization and prevent macular edema.
Indications for the use of aflibercept: wet form of macular degeneration, macular edema associated with retinal vein thrombosis, diabetic retinopathy.