The Ahmed valve is a device designed to control intraocular pressure that is used in the surgical treatment of all types of glaucoma. Its implantation helps to significantly reduce the amount of medications consumed, and also reduces the risk of hypotension.
The main element of the valve, or rather, this drainage system, is a unique membrane that causes the valve to open when the intraocular pressure in the anterior chamber of the eye exceeds normal. The tubules on its posterior surface provide communication between the anterior chamber and the sub-Tennon's space, allowing the outflow of fluid. The valves located inside the system are particularly sensitive to pressure, which regulates the flow.
The operation is recognized as the most effective method of surgical treatment of glaucoma. However, it can only be performed by very experienced specialists, due to the high risk of complications.
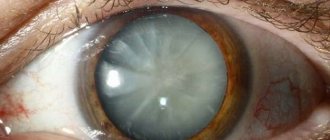
Glaucoma
Glaucoma Source: dvaglaza.ru
Glaucoma is a set of disorders caused by obstructed outflow of fluid from the anterior chamber of the eye. Over time, increased intraocular pressure leads to organic changes in the tissues of the optic nerve, causing its atrophy.
In the absence of treatment or its ineffectiveness, glaucoma leads to complete blindness, since sensitive tissues, due to constant disruption of trophism, die without the possibility of recovery.
Glaucoma is a progressive disease that leads to irreversible blindness. Due to increased intraocular pressure in glaucoma, retinal cells are destroyed, the optic nerve atrophies, and visual signals stop entering the brain.
Currently, glaucoma is understood as a fairly large group of diseases, often of different origins and with different courses. There is still no consensus on what causes the development of these ailments, but in the absence of treatment, their outcome is the same - optic nerve atrophy and blindness.
Ahmed valve - implantation for glaucoma
Source: glaucomacentr.ru
Drainage surgery is a separate area of general ophthalmic surgery, whose operations are aimed at improving the results of traditional surgical interventions through the implantation of certain drainages. Such drains prevent excessive scarring and prevent blocking of the formed outflow pathways.
Drainage implants are plastic devices that maintain a connection between the anterior chamber of the eye and its sub-Tenon's space. Some types of modern drainages are designed like a tube, which serves for the outflow of intraocular fluid.
Others do not have an internal opening and the intraocular fluid flows along their surface. There are also those whose design is quite complex, these are drains with a valve (Ahmed, Krupin) and those without a valve (Molteno, Baerveldt).
Such drains are sensitive to changes in pressure and, in response to it, regulate filtration. And yet, regardless of the type of drainage, all devices are designed to solve one problem - to reduce intraocular pressure by improving outflow.
In 1907, Rollet made a measured attempt to reduce IOP by draining aqueous humor and the subconjunctival space through the limbus by implanting horsehair.
Interesting to know
A breakthrough in this field occurred in 1969, when Dr. Anthony Molteno proposed using the large surface of the eyeball near the limbus to distribute aqueous humor.
The effectiveness of operations increased in 1973, when Molteno began to drain aqueous humor to an area located 8-10 mm from the limbus. To date, all anti-glaucoma drainages are based on the Molteno concept.
The first anti-glaucoma drainages did not take into account any resistance to the outflow of aqueous humor from the anterior chamber. Among these valveless drains, the most commonly used are the Molteno drain, proposed by Anthony Molteno in 1969, and the Baerveldt drain, proposed by George Baerveldt in 1992.
The Ahmed valve includes tubes with silicone membranes that open at a pressure of 10-12 mm Hg. Art. The implantation of such a device has been proven to be highly effective for complicated and refractory glaucoma. This drainage system reduces the need for constant use of medications in the following cases:
- simultaneous aphakia and glaucoma;
- pseudophakia (artificial lens);
- uveal glaucoma;
- increased IOP after vitreoretinal surgery;
- with neovascular glaucoma;
- after penetrating keratoplasty.
The implanted valve connects the anterior chamber with the sub-Tenon's space through tubes and ensures the removal of excess fluid only at a certain level of IOP. As soon as the pressure drops to normal, the valve membrane closes the lumen of the channels and stops the outflow.
This valve, through tubules on the posterior surface, connects the anterior chamber with the sub-Tennon's space. Valves located inside are pressure sensitive and regulate flow. The operation should only be performed by experienced specialists, as complications may develop.
The main element of the valve, or rather, this drainage system, is a unique membrane that causes the valve to open when the intraocular pressure in the anterior chamber of the eye exceeds normal.
The tubules on its posterior surface provide communication between the anterior chamber and the sub-Tennon's space, allowing the outflow of fluid. The valves located inside the system are particularly sensitive to pressure, which regulates the flow.
Indications include:
- Ineffective surgical treatment of secondary glaucoma after trabeculectomy with or without the use of antimetabolites (aniridia, traumatic and neovascular glaucoma).
- Decompensated glaucoma after trabeculectomy with antimetabolites.
- Significant cicatricial changes in the conjunctiva after its excision.
- Ineffective operations (trabeculectomy, trabeculotomy, goniotomy) for congenital glaucoma.
The Ahmed valve contains a scleral explant that forms a filtration cushion. The distal end of the shunt is located in the anterior chamber of the eye, through which the fluid flows into the area around the explant (10-12 mm from the limbus).
As pressure increases, there is a passive outflow of liquid contents and normalization of indicators, which depend on the thickness of the capsule wall and the encapsulation area.
Indications for surgery
The surgical procedure to implant the Ahmed valve is performed for the following conditions:
- Lack of a positive result after surgery to remove trabeculae for secondary glaucoma with or without the use of antimetabolites (aniridia, neovascular or traumatic glaucoma).
- Decompensation of glaucoma after removal of trabeculae during the use of antimetabolites.
- Serious cicatricial defects of the conjunctiva after its excision.
- Unsuccessful surgical interventions for congenital glaucoma (trabeculectomy, trabeculotomy, goniotomy).
• Uncompensated glaucoma after trabeculectomy using antimetabolites.
• Operated secondary glaucoma after trabeculectomy with or without antimetabolites, which was not successful (neovascular glaucoma, aniridia and glaucoma after anterior segment injury).
MORE ABOUT: Doxazosin reviews for men effect on potency
• Pronounced cicatricial changes in the conjunctiva, eliminated by careful and thorough excision.
• Some types of congenital glaucoma after unsuccessful surgical interventions (goniotomy, trabeculotomy, trabeculectomy).
Implantation of drains is not performed in all cases of antiglaucomatous operations. The following indications exist for their installation:
- Lack of compensation for glaucoma after trabeculectomy with the use of antimetabolites.
- Secondary operated glaucoma that was not successful after trabeculectomy with or without antimetabolites (traumatic, neovascular and anirid glaucoma).
- Pronounced cicatricial transformation of the conjunctiva, with the possibility of its careful excision.
- Unsuccessful surgical treatment of some types of congenital glaucoma (trabeculotomy, trabeculectomy, goniotomy).
Types and types of drainage
Molteno, Baerveldt, Krupin and Ahmed implants (Ahmed valve) are used. The latter is the most commonly used and popular. The design of all implants is based on the original technical solution of Molteno drainage.
The implant has a scleral explant, the task of which is to create a functioning filtration cushion. The free end of the hollow tube, a shunt for the outflow of intraocular fluid, is placed in the anterior chamber of the eye. Outflow is carried out into an encapsulated area around the explant, which remains 10-12 mm posterior to the limbus.
Intraocular pressure decreases passively, in accordance with the level of IOP, through the outflow of intraocular fluid through the wall of the drainage capsule. The dynamics of the pressure level decrease is directly related to the outflow resistance, as well as the total encapsulation area.
Currently, tubular implants are divided into two types:
- valveless (for example Molteno, Baerveldt, Schocket)
- valve (for example, Krupin, Ahmed).
Basic requirements for anti-glaucoma drainages
- the ability to remove aqueous humor from the anterior chamber without developing hypotension
- duration of hypotensive effect with minimal side effects on surrounding tissues
Valveless implants, such as Molteno and Baerveldt drains, do not take into account outflow resistance, which can lead to hypotony, the formation of a shallow anterior chamber and ciliochoroidal detachment.
To avoid these complications, several drainage modifications have been proposed:
- applying an occluding ligature to the tube for a certain time;
- packing the tube with a material that slowly absorbs intraocular fluid;
- insertion of a thread into the tube, which can later be removed.
The Krupin drain valve consists of a slit-shaped silicone valve attached to a silicone plate. As the IOP rises, the valve opens, allowing aqueous humor to leak out.
Model S2, the prototype of the Ahmed valve, features silicone diaphragms folded together and extended at the top. The membranes are based on polypropylene plates that have additional ridges and grooves that stretch the membranes.
Since the valve begins to work only at a pressure above 8-10 mm Hg. Art., this prevents the development of hypotension. Unlike valveless devices, the Ahmed valve is implanted in one stage, and IOP decreases immediately after surgery.
Among all tubular implants, the Ahmed valve has the lowest postoperative rate of hypotension and few complications. The internal resistance in the valve system is provided by the Venturi mechanism. The valve has an inlet area larger than the outlet area.
The Ahmed valve is a non-obstructive device. Since the valves are larger than the circumference of the narrow tube, every particle that passes through the tube also passes through the valve mechanism.
- Ahmed valve prototype: Model S2
Ahmed's first valve was the S2. The device consists of a polypropylene plate on which a valve mechanism with an area of 184 mm2 is mounted. After the formation of a filtration cushion over the body of the device, fluid drainage mainly occurs through the vascularized tissue of the capsule.
The length of the silicone tube is 25 mm, the internal diameter is 0.635 mm; device width -13 mm, length - 16 mm, thickness - 1.9 mm. Thin silicone membranes are 8 mm long and 7 mm wide.
- Various biomaterials and scar tissue formation: model FP7
The original Model S2 glaucoma valve uses a polypropylene plate. Ayyala et al. found that polypropylene has lower biocompatibility compared to silicone.
The use of silicone can reduce the degree of inflammation and scarring during device encapsulation, and the severity of encapsulation affects the duration and severity of the hypertensive phase.
The result of this research was the release of silicone implants by New World Medical, including the Ahmed valve on an elastic plate (model FP7).
The thin profile of the implant also avoids the need for posterior release incisions or the use of conjunctival autograft.
- Drains: models B1 and B4, FX1 and FX4
Long-term results of studies have shown a direct dependence of the reduction in IOP on the size of the implant, but only if its area is sufficient. Baerveldt implants with an area of 250, 350 and 500 mm2 are located partially under the rectus muscles, protruding only slightly, and act as a moisture distribution reservoir.
Britt, in a prospective study comparing Baerveldt 350 mm2 and 500 mm2 implants, found no statistically significant difference in IOP reduction over 18 months. observations after their use.
Similar results were obtained by Ayyala et al. when comparing the Molteno double-plate drainage with the Ahmed single-chamber valve. These data support the view that increasing implant surface area increases antihypertensive effectiveness only up to a certain size.
A second plateau (model B4) can also be attached to the valve to the right and left of it if it is desired to reduce IOP by increasing the surface area. Model B4 consists of a 14.8 mm long silicone drainage tube attached to a 12.2 mm wide polypropylene plate with a surface area of 180 mm2.
The second plateau can be sutured in the quadrant next to the base of the valve, after which the wall of the filtration pad is punctured with a tube that does not have a valve and the plateau is fixed.
Thus, the filtration pad is connected via a tube to the second valveless plateau. Recently, these devices began to be made of silicone (models FX1 and FX4).
Clinical indications and contraindications
Until recently, Ahmed valves were widely used for refractory glaucoma when previous trabeculectomy had failed or was unlikely to be effective with or without antimetabolic agents.
Ahmed valves can be used for advanced congenital, aphakic or pseudophakic open-angle glaucoma, closed-angle glaucoma, glaucoma induced by keratoprosthesis, penetrating keratoplasty, trauma, aniridia, Sturge-Weber syndrome and other types of secondary glaucoma.
Many surgeons use Ahmed valves as a primary intervention in cases of neovascular, congenital, uveal or open-angle glaucoma, in the presence of perilimbal scarring or anterior synechiae.
Valvular devices are often used as a last resort treatment option after unsuccessful trabeculectomy, but this view is now changing. When compared with trabeculectomy, valve implants have fewer late complications associated with filtration cushions (eg, external filtration, phlebitis, and endophthalmitis).
The lower incidence of endophthalmitis may be associated with the location of the base of the valve more distant from the limbus (8-10 mm), where the subconjunctival tissue is thicker and the fibroblastic reaction is more pronounced.
These tissues are different from trabeculectomy filter pads, which are more likely to cause phlebitis and endophthalmitis, especially when mitomycin C and 5-fluorouracil are used.
Childhood glaucoma is a group of diseases that can potentially lead to blindness and are often resistant to drug therapy, so Ahmed valve implantation is often indicated in such situations.
The advantages of the Ahmed FP7 silicone valve are one-stage implantation, intervention in only one quadrant and instant IOP control. This is especially important in cases where urgent combined intervention is needed for corneal disease in severe congenital glaucoma.
Indications for implantation
The Ahmed S2 valve can be used in both children and adolescents. It is used in the absence of IOP compensation, despite maximum drug therapy and laser intervention. This valve can be used as a primary intervention in cases of neovascular, postveal types of secondary glaucoma.
A second valveless model (Model B1) can be implanted later if necessary to improve fluid drainage through the primary filtration pad.
The tube extension (model TE) is used in the following situations:
- when the tube is pushed out or exposed;
- when the surgeon wants to move the valve from the pars plana of the ciliary body by inserting a tube into the anterior chamber;
- when the tube is cut too short during implantation;
- in all cases where the tube is accidentally cut.
Attachment in the pars plana area (model PC) with insertion of a tube through it is advisable in patients after keratoplasty or with a modified cornea. Drainage is also used in patients with a very shallow anterior chamber or when it is impossible to insert a tube into it due to anterior synechiae.
Implantation of valves in the pars plana area is often performed for advanced secondary glaucoma with an uncompensated IOP level.
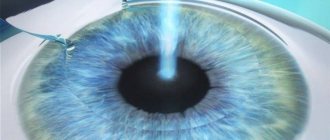
Operating technique using the Ahmed valve
Source: mosglaz.ru
It is necessary to obtain the informed consent of the patient for surgery with implantation of the Ahmed valve and strengthening of the sclera using pre-prepared pericardium (Tutoplast), donor sclera, etc.
If a tube is planned to be inserted through the pars plana, a retinal examination is necessary. If indicated, implantation of the Ahmed valve can be combined with vitrectomy through the pars plana.
Progress of the operation
In addition to retro- or peribulbar anesthesia, topical tetracaine can be used. After applying a frenulum suture (6/0 polyglactin suture), a special shield is placed on the cornea to protect it from bright light from the microscope.
The Ahmed valve should be flushed with a balanced salt solution to ensure that all air has been removed and the valve is functioning.
To do this, a syringe with a 26G needle or a 27G cannula is inserted into the tubing and the plunger of the balanced salt solution syringe is pressed firmly until the solution is visible behind the valve or at the top of its base.
The preferred location for implantation is the upper outer quadrant. The device should not be implanted in the upper inner quadrant because this is associated with a high risk of developing pseudo-Brown syndrome and damage to the superior oblique tendon.
A conjunctival flap, base to fornix, is formed between two adjacent rectus muscles. It is convenient to use Stevens scissors for this purpose.
The Ahmed valve should not be grabbed by its body with tweezers, because its damage will promote the growth of fibrovascular tissue. The device must be taken by the base next to the small hole, inserted into the formed subconjunctival space and sutured to the sclera with non-absorbable sutures.
When suturing the plateau to the sclera, it is necessary to ensure that the valve is located between the rectus muscles and the tube is directed radially in relation to the limbus. The front edge of the valve should be 8-10 mm from the dial.
Recommendation
When implanting an Ahmed valve with two plateaus, it is recommended to create a flap with the base towards the vault. A traction suture on Tenon's capsule with 7/0 polypropylene suture or 8/0 nylon suture may be helpful.
The connecting tube is placed above or below the superior rectus muscle, but there should be no Tenon's capsule tissue underneath the connecting tube. The second plateau, which is also located 8-10 mm from the limbus, should be attached to the sclera with a 9/0 nylon thread.
The length of the drainage tube is calculated based on its location outside the cornea, ensuring that the length is sufficient for insertion into the anterior chamber. The drainage tube is then cut obliquely so that the beveled end is directed toward the corneal endothelium.
Then paracentesis is performed outside the surgical area. If necessary, viscoelastic is injected into the anterior chamber. Using a 23G needle, enter the anterior chamber in the corneoscleral region parallel to the plane of the iris. Using special tweezers, the tube is passed into the anterior chamber through a puncture made with a needle.
The length of the tube in the anterior chamber should not be more than 2-3 mm and should not touch the cornea, iris or lens. The entrance hole is covered with donor sclera or pericardium, which is sutured to the sclera with a 9/0 polyglactin or nylon suture on a flat or TG needle.
The conjunctiva is sutured with absorbable sutures (eg, 8/0 polyglactin suture). Antibiotics and corticosteroids are administered subconjunctivally 180° from the intervention site.
In some countries where donor tissue is not available, alternative methods are used to close the exposed tube. One of them is the formation of a scleral flap with the base towards the limbus for part of the thickness of the sclera.
Then, using a 23G needle, a hole is created under the flap for insertion of the tube. The tube is covered with a flap, which is then sutured with 9/0 nylon suture. After suturing the conjunctiva, antibiotics and corticosteroids are administered subconjunctivally.
Implantation in the pars plana of the ciliary body
After the formation of the conjunctival flap with its base towards the fornix, the Ahmed valve with fixation to the flat part of the ciliary body (purs plana) is washed through the PS irrigation cannula. The valve is placed 6-8 mm from the limbus and fixed to the sclera with a 9/0 nylon thread. It is better and easier to fix the valve plateau to the sclera while the eye is still tight.
If necessary, vitrectomy through the pars plana can be performed, which will increase the hypotensive effect of drainage surgery if the main part of the vitreous is removed from the area where the drainage device is implanted. After vitrectomy, you can use an infusion of a solution to restore normal eye turgor.
The valve drain is then inserted through a puncture made with a 23-gauge needle approximately 3.5 mm from the limbus. The tube can be cut so that the end reaches the edge of the pupil, allowing it to be seen later at the slit lamp.
Bending of the tube is eliminated by passing it through the plateau with fastening in the flat part of the ciliary body. If a plate with fasteners for connecting to other drains is included separately in the kit (PC model), the surgeon must pull the tube through it with fine tweezers.
Unlike other drainages, the advantage of a plateau with fastening in the area of the flat part of the ciliary body is the mobility of the plateau, allowing it to be positioned at any distance from the valve plateau. After strengthening the sclera with donor material (sclera or pericardium), the port and conjunctiva are sutured.
Ahmed valve in glaucoma surgery
- Valve implants
- Clinical indications and contraindications
- Operation technique
- results
- Complications
In 1907, Rollet made a measured attempt to reduce IOP by draining aqueous humor and the subconjunctival space through the limbus by implanting horsehair. After this, many attempts were made, most often unsuccessful, to create drainage devices from various materials. All of these surgeries failed due to excessive scarring in the surgical area.
A breakthrough in this field occurred in 1969, when Dr. Anthony Molteno proposed using the large surface of the eyeball near the limbus to distribute aqueous humor. Most operations were unsuccessful after 3-6 months due to ulceration of the conjunctiva over the drainage, exposure of the plate and scarring processes. The effectiveness of operations increased in 1973, when Molteno began to drain aqueous humor to an area located 8-10 mm from the limbus. To date, all anti-glaucoma drainages are based on the Molteno concept.
The first anti-glaucoma drainages did not take into account any resistance to the outflow of aqueous humor from the anterior chamber. Among these valveless drains, the most commonly used are the Molteno drain, proposed by Anthony Molteno in 1969, and the Baerveldt drain, proposed by George Baerveldt in 1992.
Anti-glaucoma drainages that appeared later began to take into account the resistance to the outflow of aqueous humor: a valve mechanism was created. Significant progress in this direction was achieved by Theodore Krupin, who created the Krupin valve in 1979; In 1993, Abdul Mateen Ahmed introduced the Ahmed valve into widespread practice.
Currently, tubular implants are divided into two types: valveless (for example, Molteno, Baerveldt, Schocket) and valved (for example, Krupin, Ahmed).
Basic requirements for anti-glaucoma drainages
- the ability to remove aqueous humor from the anterior chamber without developing hypotension
- duration of hypotensive effect with minimal side effects on surrounding tissues
Valveless implants, such as Molteno and Baerveldt drains, do not take into account outflow resistance, which can lead to hypotony, the formation of a shallow anterior chamber and ciliochoroidal detachment. To avoid these complications, several modifications of drains have been proposed: applying an occluding ligature to the tube for a certain time; packing the tube with a material that slowly absorbs intraocular fluid; insertion of a thread into the tube, which can later be removed.
Valve implants
The Krupin drain valve consists of a slit-shaped silicone valve attached to a silicone plate. As the IOP rises, the valve opens, allowing aqueous humor to leak out. The length and width of the slit and the elasticity of the tube help control the outflow of aqueous humor. This is an obstructive type valve, i.e. To enhance the outflow, it is necessary to open a slit in the tube.
Model S2, the prototype of the Ahmed valve, features silicone diaphragms folded together and extended at the top. The membranes are based on polypropylene plates that have additional ridges and grooves that stretch the membranes. When IOP becomes above 8-10 mm Hg. Art., silicone membranes open to allow fluid to drain out. The valve resistance is self-regulated by the IOP level. When the initial pressure in the anterior chamber is high, the valve automatically opens fully. As the pressure decreases, the membranes decrease in size, reducing the outflow of fluid. Since the valve begins to work only at a pressure above 8-10 mm Hg. Art., this prevents the development of hypotension.
Unlike valveless devices, the Ahmed valve is implanted in one stage, and IOP decreases immediately after surgery. Among all tubular implants, the Ahmed valve has the lowest postoperative rate of hypotension and few complications. The internal resistance in the valve system is provided by the Venturi mechanism. The valve has an inlet area larger than the outlet area.
The Ahmed valve is a non-obstructive device. Since the valves are larger than the circumference of the narrow tube, every particle that passes through the tube also passes through the valve mechanism.
Ahmed valves are now available in a variety of designs, shapes, materials and surface areas. They also come with useful add-ons that can be used on both valved and valveless drains. This brief history of the evolution of Ahmed valves describes the key concepts that have had a major impact on the development of glaucoma drainage devices, and also highlights important issues for clinicians, such as choosing the right model for a particular clinical situation.
Ahmed valve prototype: Model S2
Ahmed's first valve was the S2. The device consists of a polypropylene plate on which a valve mechanism with an area of 184 mm2 is mounted. After the formation of a filtration cushion over the body of the device, fluid drainage mainly occurs through the vascularized tissue of the capsule. The length of the silicone tube is 25 mm, the internal diameter is 0.635 mm; device width -13 mm, length - 16 mm, thickness - 1.9 mm. Thin silicone membranes are 8 mm long and 7 mm wide.
Various biomaterials and scar tissue formation: model FP7
The original Model S2 glaucoma valve uses a polypropylene plate. Ayyala et al. found that polypropylene has lower biocompatibility compared to silicone. The use of silicone can reduce the degree of inflammation and scarring during device encapsulation, and the severity of encapsulation affects the duration and severity of the hypertensive phase. The result of this research was the release of silicone implants by New World Medical, including the Ahmed valve on an elastic plate (model FP7).
These implants are more flexible, with thinner edges (60% less than the S2 model). According to Sarkisian et al., a thin lower edge facilitates suturing of the conjunctiva, reduces the incidence of conjunctival perforation, promotes better healing and better formation of the filtration cushion. The thin profile of the implant also avoids the need for posterior release incisions or the use of conjunctival autograft.
The elastic plate has three holes located behind the valve and outside or between two support elements. The holes are made to allow fluid to drain through them onto the top of the implant, thereby increasing the efficiency of aqueous humor drainage. Moreover, fibrous tissue, growing through these holes, helps to reduce the height of the filtration cushion. Thanks to these innovations, the Ahmed FP7 valve is able to provide better IOP control.
Drains: models B1 and B4, FX1 and FX4
Long-term results of studies have shown a direct dependence of the reduction in IOP on the size of the implant, but only if its area is sufficient. Baerveldt implants with an area of 250, 350 and 500 mm2 are located partially under the rectus muscles, protruding only slightly, and act as a moisture distribution reservoir.
Britt, in a prospective study comparing Baerveldt 350 mm2 and 500 mm2 implants, found no statistically significant difference in IOP reduction over 18 months. observations after their use.
Similar results were obtained by Ayyala et al. when comparing the Molteno double-plate drainage with the Ahmed single-chamber valve. These data support the view that increasing implant surface area increases antihypertensive effectiveness only up to a certain extent, and further increasing area does not provide additional benefit.
To increase the area, New World Medical has released an implant with two plateaus (model B1). The total surface area of such an implant was 364 mm2.
A second plateau (model B4) can also be attached to the valve to the right and left of it if it is desired to reduce IOP by increasing the surface area. Model B4 consists of a 14.8 mm long silicone drainage tube attached to a 12.2 mm wide polypropylene plate with a surface area of 180 mm2.
The second plateau can be sutured in the quadrant next to the base of the valve, after which the wall of the filtration pad is punctured with a tube that does not have a valve and the plateau is fixed. Thus, the filtration pad is connected via a tube to the second valveless plateau. Recently, these devices began to be made of silicone (models FX1 and FX4).
Ahmed valves for pediatric practice: models S3, FP8
The Ahmed S3 valve for children has a reduced size and silicone plate (model FP8) with a base area of 96 mm2, a length of 10 mm and a width of 9.6 mm.
Tube extension: Model TE
The tube extender is a simple silicone device that can be used for all current drains (Molteno, Baerveldt, Krupin and Ahmed). In situations where it is necessary to change the length of the valve tube, an extension is the ideal solution. It consists of three parts.
- The first part connects the valve tube to the extension tube.
- The second part has two holes for fixing the tube extension to the sclera after tightly connecting the valve tube to the extension.
- The third part of the extension is the same size as the valve tube.
Tube extension dimensions: height - 1.14 mm, width - 3.05 mm, length - 24 mm. It can be sutured to the sclera with 8/0 or 9/0 vicryl sutures. A tube extender is a useful device in particularly difficult situations.
Mounting in the flat part of the ciliary body: models PC, PS2 and PS3, PC7 and PC8
Implanted tubes for glaucoma, inserted at an acute angle into the posterior chamber of the eye, tend to bend. With this in mind, the PC model with a flat ciliary body attachment has a hole in the center to guide the tube through the pars plana.
The attachment for the flat part of the ciliary body is made of silicone. Its dimensions: width - 4.6 mm, length - 5.6 mm. For hemming, there are two 0.025 inch diameter holes and a center hole with an internal diameter of 0.635 mm. The fasteners are designed so that they can be placed at any distance from the valve. The mounts are available either separately (model PC) or in conjunction with a polypropylene plate (models PS2, PS3) or silicone plate (models PC7 and PC8).
Tweezers for fixing the drainage tube model TI
To grasp the tube extension and facilitate its insertion into the anterior chamber, a special forceps has been created that can be used for all implants.
Clinical indications and contraindications
Until recently, Ahmed valves were widely used for refractory glaucoma when previous trabeculectomy had failed or was unlikely to be effective with or without antimetabolic agents. Ahmed valves can be used for advanced congenital, aphakic or pseudophakic open-angle glaucoma, closed-angle glaucoma, glaucoma induced by keratoprosthesis, penetrating keratoplasty, trauma, aniridia, Sturge-Weber syndrome and other types of secondary glaucoma.
Many surgeons use Ahmed valves as a primary intervention in cases of neovascular, congenital, uveal or open-angle glaucoma, in the presence of perilimbal scarring or anterior synechiae. For these types of glaucoma, implantation of Ahmed valves increases the success of surgical treatment.
Valvular devices are often used as a last resort treatment option after unsuccessful trabeculectomy, but this view is now changing. When compared with trabeculectomy, valve implants have fewer late complications associated with filtration cushions (eg, external filtration, phlebitis, and endophthalmitis). The lower incidence of endophthalmitis may be associated with the location of the base of the valve more distant from the limbus (8-10 mm), where the subconjunctival tissue is thicker and the fibroblastic reaction is more pronounced. These tissues are different from trabeculectomy filter pads, which are more likely to cause phlebitis and endophthalmitis, especially when mitomycin C and 5-fluorouracil are used.
Childhood glaucoma is a group of diseases that can potentially lead to blindness and are often resistant to drug therapy, so Ahmed valve implantation is often indicated in such situations. This provides significantly greater effectiveness in stabilizing the glaucomatous process during the first 2 years of life compared to trabeculectomy using mitomycin C. The advantages of the Ahmed FP7 silicone valve are one-stage implantation, intervention in only one quadrant and instant IOP control. This is especially important in cases where urgent combined intervention is needed for corneal disease in severe congenital glaucoma.
Indications for implantation
The Ahmed S2 valve can be used in both children and adolescents. It is used in the absence of IOP compensation, despite maximum drug therapy and laser intervention. This valve can be used as a primary intervention in cases of neovascular, postveal and other complicated types of secondary glaucoma.
A second valveless model (Model B1) can be implanted later if necessary to improve fluid drainage through the primary filtration pad.
The tube extension (model TE) is used in the following situations:
- when the tube is pushed out or exposed;
- when the surgeon wants to move the valve from the pars plana of the ciliary body by inserting a tube into the anterior chamber;
- when the tube is cut too short during implantation;
- in all cases where the tube is accidentally cut.
Attachment in the pars plana area (model PC) with insertion of a tube through it is advisable in patients after keratoplasty or with a modified cornea. Drainage is also used in patients with a very shallow anterior chamber or when it is impossible to insert a tube into it due to anterior synechiae. Implantation of valves in the pars plana area is often performed for advanced secondary glaucoma with an uncompensated IOP level.
Operation technique
It is necessary to obtain the informed consent of the patient for surgery with implantation of the Ahmed valve and strengthening of the sclera using pre-prepared pericardium (Tutoplast), donor sclera, etc. If a tube is planned to be inserted through the pars plana, a retinal examination is necessary. If indicated, implantation of the Ahmed valve can be combined with vitrectomy through the pars plana.
Progress of the operation
In addition to retro- or peribulbar anesthesia, topical tetracaine can be used. After applying a frenulum suture (6/0 polyglactin suture), a special shield is placed on the cornea to protect it from bright light from the microscope. The Ahmed valve should be flushed with a balanced salt solution to ensure that all air has been removed and the valve is functioning. To do this, a syringe with a 26G needle or a 27G cannula is inserted into the tubing and the plunger of the balanced salt solution syringe is pressed firmly until the solution is visible behind the valve or at the top of its base.
The preferred location for implantation is the upper outer quadrant. The device should not be implanted in the upper inner quadrant because this is associated with a high risk of developing pseudo-Brown syndrome and damage to the superior oblique tendon. A conjunctival flap, base to fornix, is formed between two adjacent rectus muscles. It is convenient to use Stevens scissors for this purpose.
The Ahmed valve should not be grabbed by its body with tweezers, because its damage will promote the growth of fibrovascular tissue. The device should be held at the base near the small opening, inserted into the formed subconjunctival space, and sutured to the sclera with nonabsorbable sutures (eg, 9/0 nylon suture). When suturing the plateau to the sclera, it is necessary to ensure that the valve is located between the rectus muscles and the tube is directed radially in relation to the limbus. The front edge of the valve should be 8-10 mm from the dial.
When implanting an Ahmed valve with two plateaus, it is recommended to create a flap with the base towards the vault. A traction suture on Tenon's capsule with 7/0 polypropylene suture or 8/0 nylon suture may be helpful. The connecting tube is placed above or below the superior rectus muscle, but there should be no Tenon's capsule tissue underneath the connecting tube. The second plateau, which is also located 8-10 mm from the limbus, should be attached to the sclera with a 9/0 nylon thread.
The length of the drainage tube is calculated based on its location outside the cornea, ensuring that the length is sufficient for insertion into the anterior chamber. The drainage tube is then cut obliquely so that the beveled end is directed toward the corneal endothelium. Then paracentesis is performed outside the surgical area. If necessary, viscoelastic is injected into the anterior chamber. Using a 23G needle, enter the anterior chamber in the corneoscleral region parallel to the plane of the iris. Using special tweezers, the tube is passed into the anterior chamber through a puncture made with a needle. The length of the tube in the anterior chamber should not be more than 2-3 mm and should not touch the cornea, iris or lens.
The entry hole through which the tube was inserted is covered with donor sclera or pericardium, which is sutured to the sclera with a 9/0 polyglactin or nylon suture on a flat or TG needle. The conjunctiva is sutured with absorbable sutures (eg, 8/0 polyglactin suture). Antibiotics and corticosteroids are administered subconjunctivally 180° from the intervention site.
In some countries where donor tissue is not available, alternative methods are used to close the exposed tube. One of them is the formation of a scleral flap with the base towards the limbus for part of the thickness of the sclera. Then, using a 23G needle, a hole is created under the flap for insertion of the tube. The tube is covered with a flap, which is then sutured with 9/0 nylon suture. After suturing the conjunctiva, antibiotics and corticosteroids are administered subconjunctivally.
Implantation in the pars plana of the ciliary body
After the formation of the conjunctival flap with its base towards the fornix, the Ahmed valve with fixation to the flat part of the ciliary body (purs plana) is washed through the PS irrigation cannula. The valve is placed 6-8 mm from the limbus and fixed to the sclera with a 9/0 nylon thread. It is better and easier to fix the valve plateau to the sclera while the eye is still tight.
If necessary, vitrectomy through the pars plana can be performed, which will increase the hypotensive effect of drainage surgery if the main part of the vitreous is removed from the area where the drainage device is implanted. After vitrectomy, you can use an infusion of a solution to restore normal eye turgor.
The valve drain is then inserted through a puncture made with a 23-gauge needle approximately 3.5 mm from the limbus. The tube can be cut so that the end reaches the edge of the pupil, allowing it to be seen later at the slit lamp. Bending of the tube is eliminated by passing it through the plateau with fastening in the flat part of the ciliary body.
If a plate with fasteners for connecting to other drains is included separately in the kit (PC model), the surgeon must pull the tube through it with fine tweezers. Unlike other drainages, the advantage of a plateau with fastening in the area of the flat part of the ciliary body is the mobility of the plateau, allowing it to be positioned at any distance from the valve plateau.
After strengthening the sclera with donor material (sclera or pericardium), the port and conjunctiva are sutured.
Postoperative management
Antibiotics and corticosteroids are prescribed topically 4 times a day. Depending on the degree of postoperative inflammation, the instillation regimen is reduced within a month. Routine postoperative restrictions are recommended.
results
Ahmed valves are used for all types of glaucoma. They showed a clear advantage over other valveless devices. The valve mechanism with the Venturi system allows you to quickly reduce IOP and avoid hyperfiltration in the postoperative period. In vitro studies tested the valve mechanism and its ability to control pressure by comparing the performance of the Krupin and Ahmed valves. Studies have shown that the Krupin valve offers the same resistance to drainage and pressure control as a cannula. Only the Ahmed valve functioned as a valve, regulating pressure within a desired range, reducing or increasing resistance to fluid flow.
Based on studies conducted in the United States and some other countries, it is cautious to say that early valve use is warranted, especially in cases of neovascular, congenital, uveal, and postkeratoplastic glaucoma.
Wang's comparative studies showed that in patients with refractory glaucoma, the 350 mm2 Baerveldt drain and the S2 Ahmed valve provided equally good control of IOP, preserving visual function, and had few complications 1 year after surgery.
Over the past three years, the use of flexible plates (model FP7) has increased 3 times compared to models S2/S3. Although there are no publications in the literature yet, many clinicians have reported the formation of tall filtration cushions with a thinner wall after implantation of the FP7 model. As expected during development, the FP7 model can provide significant IOP reduction compared to the S2 model. A low filtration cushion reduces the number of complications such as diplopia and strabismus. Moreover, the use of silicone causes less inflammatory reaction and reduces the thickness of the pseudocapsule.
The new silicone valve FP7 has the advantage of one-stage implantation in one quadrant with an immediate reduction in IOP, which is very important in neovascular glaucoma. Also, implantation of the Ahmed valve is a safe and effective operation for uveal glaucoma in children, especially with normal immune status and under close supervision.
In patients with glaucoma who require penetrating keratoplasty, implantation of Ahmed valve drainage promotes longer transparent engraftment of the graft by reducing IOP and reducing the number of installed drugs. The use of drainage surgery, according to Kwon et al., ensures engraftment of the corneal graft in 70 and 55% of cases during follow-up for 2 and 3 years, respectively, and allows normalization of IOP levels within 3 years in the majority (82%) of patients after penetrating keratoplasty.
Complications
Hyperfiltration of aqueous humor is one of the early postoperative complications leading to a shallow anterior chamber, hypotension and ciliochoroidal detachment. Ahmed valve drainage has a protective mechanism that minimizes hyperfiltration of aqueous humor in the early postoperative period. Drainage implants without this mechanism (for example, Baerveldt and Molteno drainages) are placed either in 2 stages or an additional suture is applied to prevent hypotony and ciliochoroidal detachment.
As a rule, a period of transient increase in IOP is observed within a month after surgery. This period is called the ocular hypertension phase. However, this does not necessarily require additional intervention. Huang et al. It is believed that an increase in IOP is possible no earlier than 4 weeks after surgery.
In children, tube exposure . This may be due to the fact that young children often rub their eyes. Because tube exposure is associated with an increased risk of endophthalmitis, surgery to extend the tube, displace the valve plateau, or replace the implant is often necessary.
Complications
Source: slideshare.net
Complications of Ahmed valve placement include:
- An increase in the volume of drainage, which leads to a decrease in the size of the anterior chamber. Correction of this can be carried out in the early postoperative period by applying adjustable sutures, which will reduce the lumen of the shunt.
- If the shunt is poorly fixed, corneal dystrophy may develop due to contact with the implant.
- If the shunt comes into direct contact with the lens, the cataract will progress.
- Dislocation of the shunt from the anterior chamber.
- Formation of trophic changes (bedsores) in the shunt area. To reduce the risk of this complication, the surface of the tube is covered with donor sclera.
- Occlusion of the shunt by a foreign body, blood clot, iris, or vitreous tissue.
- Double vision occurs when the joint functioning of the extraocular muscles is disrupted. More often this happens when the valve body is placed in the area of the rectus muscle or when the surface area of the implant is large.
- Formation of a dense capsule around the filtration cushion, which develops over time in 10% of patients.
- Late endophthalmitis.
Hyperfiltration of aqueous humor is one of the early postoperative complications leading to a shallow anterior chamber, hypotension and ciliochoroidal detachment.
As a rule, a period of transient increase in IOP is observed within a month after surgery. This period is called the ocular hypertension phase. However, this does not necessarily require additional intervention. Huang et al. It is believed that an increase in IOP is possible no earlier than 4 weeks after surgery.
In children, tube exposure is more common. This may be due to the fact that young children often rub their eyes. Because tube exposure is associated with an increased risk of endophthalmitis, surgery to extend the tube, displace the valve plateau, or replace the implant is often necessary.
Results of the operation
Efficiency
The first improvements are already noticeable immediately after the installation of the Ahmed regulation device, but visual acuity is not restored, but is maintained at the same level. For the entire service life of the device, provided that it is initially correctly installed, the patient is not bothered by the symptoms of glaucoma, and the development of the disease is suspended.
Complications
- Excessive fluid drainage with shallowing of the anterior chamber of the eye. The problem is solved in the early rehabilitation period by adjusting the stitches.
- Injury to the cornea due to poor fixation of the tube with its effect on the endothelium.
- Decompensation of cataracts due to friction of the tube on the lens.
- Loss due to short drainage length.
- Bedsores of the conjunctiva.
- Disturbances in the outflow of the tube due to blockage by the vitreous body, iris tissue, and blood.
- Diplopia. Develops when the size of the valve or the placement of the device under the eye muscle is inappropriate.
- Isolation of the filtration pad due to poor fluid outflow through the drainage.
When the valve or its drainage tube is displaced, there is a risk of disruption of the device, which can lead to difficulty moving the eye and decompensation of the disease. In this case, an operation is required to restore the position of the device. There is also a risk of developing late endophthalmitis, an inflammation with accumulation of pus in the eye. To avoid complications, implantation should be performed by an experienced specialist.
Outcomes
Treatment results are directly related to the type of glaucoma and the degree of IOP increase. If the latter indicator is less than 21 mm Hg. Art., the effectiveness of taking local drugs reaches 50-70%. If additional drug therapy is not used, the valve is effective only in a third of cases.
In neovascular glaucoma, the effectiveness is quite low due to the progressive destruction of the retina and atrophy of the eyeball with impaired visual function. To increase the effectiveness of using the Ahmed valve during surgery, mitomcin is additionally used.
The Ahmed valve is installed surgically. Like any surgical intervention, implantation of a drainage system carries certain risks. In this case, the following complications are most likely:
- Reduction in the size of the anterior chamber due to an increase in the volume of drainage (removed by applying sutures and adjusting the lumen of the shunt).
- Corneal dystrophy due to weak fixation of the shunt and its constant contact with the implant.
- Changing the position of the shunt (falling out of the anterior chamber).
- Dystrophy up to bedsores in places of contact with the shunt (coating the shunt with donor sclera reduces the likelihood of tissue contact with artificial material and helps maintain normal trophism).
- Shunt occlusion. The lumen of the drainage tubes can be blocked by a blood clot, foreign body, vitreous tissue or iris.
- A disorder of the conjugate functioning of the oculomotor muscles, expressed by double vision and other visual distortions.
- In 10% of cases, after installing the valve, a dense capsule of connective tissue forms over time around the filtration pad.
- Endophthalmitis of the late postoperative period.
Ahmed valves are used for all types of glaucoma. They showed a clear advantage over other valveless devices. The valve mechanism with the Venturi system allows you to quickly reduce IOP and avoid hyperfiltration in the postoperative period.
In vitro studies tested the valve mechanism and its ability to control pressure by comparing the performance of the Krupin and Ahmed valves. Studies have shown that the Krupin valve offers the same resistance to drainage and pressure control as a cannula.
The effectiveness of surgery for glaucoma
We can talk about the absolute effectiveness of the Ahmed valve, which does not require further therapeutic support with drugs, in 30% of cases.
The effectiveness of implantation largely depends on the initial indicators, type and stage of glaucoma at which treatment was performed. If the IOP level did not exceed 21 mm Hg. Art., then 50-70% of patients experience sustained improvement after surgery.
Intraocular pressure is kept at a safe level, glaucoma does not progress. The required volume of local antiglaucoma drugs is significantly lower.
In the case of neovascular glaucoma, surgery is less effective due to ongoing atrophy of the eyeball and progressive destruction of the retina, accompanied by impaired visual function. Additionally, the antimetabolite Mitomycin C used during the operation increases the efficiency of the Ahmed valve.
Glaucoma is a dangerous chronic eye disease that is characterized by a constant or periodic increase in intraocular pressure (IOP). If left untreated, glaucoma causes irreversible loss of visual function up to and including complete blindness. Vision lost due to glaucoma is not restored.
With glaucoma, there is a violation of the circulation of intraocular fluid, an imbalance between its inflow and outflow, as a result of which IOP increases. Increased IOP puts pressure on the optic nerve and gradual death of its nerve fibers occurs (optic nerve atrophy).
This inevitably leads to a narrowing of the visual fields: both central and peripheral (lateral). The goal of any glaucoma treatment technique is to normalize IOP. Today, there are three types of treatment for this disease: medication, laser and surgery.
What do doctors recommend after surgery to install the Ahmed valve?
If the patient is interested in a positive outcome of treatment, then he must follow certain rules:
- after the patient is operated on, he needs to remain in a supine position for several hours, if possible, without making movements;
- You can shower after 7 days;
- do not touch your eyes with your hands;
- You should sleep on your side, with the affected eye facing up;
- Do not lift weights exceeding 5 kg for at least a month;
- strictly follow the ophthalmologist’s prescriptions, do not ignore the drops;
- without obtaining permission from a doctor to visit the pool or bathhouse.
- buy large dark glasses - do not go outside without them;
- It is prohibited to drive a car during the rehabilitation period.
The recovery period lasts approximately a month. If there are no complications, you can start doing gymnastics, gradually loading yourself.
You may be interested in: Surgery for glaucoma: indications and contraindications