Embryology
The retina is a derivative of the brain; it develops from the wall of the optic vesicle, which separates from the expansion of the diencephalon. At the end of the 4th - beginning of the 5th week of the embryonic period, invagination of the optic vesicle occurs and the formation of the optic cup, consisting of two layers (outer and inner) of neuroblastic cells. The apical surfaces of these layers are in contact with each other, and their basal surfaces face the primary choroid and the primary vitreous body. Subsequently, the outer layer of optic cup cells differentiates into the retinal pigment epithelium, in which melanin granules are formed, and the remaining layers of the retina develop from the inner layer. At this stage, there is a high mitotic activity of the cells of the inner layer of the optic cup, resulting in the formation of a multinucleated outer zone (primitive nuclear layer) and a nuclear-free inner zone. At the 5th week of the embryonic period, the primitive nuclear layer is divided into inner and outer nuclear (neuroblastic) layers; between them a transient reticular layer (Shievich) is determined. From the inner nuclear layer, consisting of 5-6 rows of cells, ganglion, amacrine and Müller cells (ganglion, amacrine neurocytes and ray gliocytes) develop, from the outer layer - photoreceptors and horizontal cells (horizontal neurocytes). Cell differentiation proceeds unevenly from the center of the cell to its periphery. The nuclear layer in the macular zone develops earlier than others, in the 3rd month of the embryonic period, the processes of the ganglion cells of this zone reach the nerve stalk in the shortest way, forming the optic nerve. At 9-10 weeks, the nuclei of rods (rod-shaped visual cells) and cones (cone-shaped visual cells) become distinguishable. Due to the processes of Müller cells, the outer limiting membrane (outer limiting layer, Babuchin membrane) is formed. By 4 months During the embryonic period, the transient reticular layer (Shievich) completely disappears; on the outside of the ganglion cells, due to their dendrites, an internal plexiform layer (inner reticular layer) is formed. During the 5th month, the photoreceptor layer moves outward due to the outer plexiform layer (outer mesh layer). The topographical features of the macula area begin to form by the 6th month of the embryonic period, but they reach full development several months after birth (usually by 6 months). The dentate line (dentate edge, T.) is formed by the 7th month of intrauterine development; by the 8th month the layer of ganglion cells and nerve fibers reaches full maturity. S.'s circulatory system begins to develop at the 4th month. Endothelial cells spread along the septa of the optic nerve, by the 6th month they form primitive vessels, in which blood flow appears. They are located at a distance of 1-2 mm from the optic nerve. By the 7th-8th month the vessels reach the dentate line. Pericytes of blood vessels become visible after 2 months. postnatal life, and the entire microcirculation system becomes similar to the microcirculation system of an adult after about 5 months. after birth.
Retinal pigment epithelium
This micrograph shows one of the most popular cell cultures of the retinal pigment epithelium - ARPE-19 (Adult Retinal Pigment Epithelial cell line-19).
This cell line was obtained in 1955 from a deceased 19-year-old man, hence the number 19 in the name. The fact is that some types of cells, when cultivated in the laboratory, are practically immortal: they quickly divide, and can do this an unlimited number of times (their telomerase prevents their telomeres from shortening, and they can be used for many years (about telomeres, see, for example, the news Chicks of old finches are born with shortened telomeres, develop quickly, die early, "Elements", 04/04/2018).The most famous of these immortal cell cultures (see Immortalized cell line) is the HeLa cervical cancer cell line, named by the first letters of the name and the surname of the donor, Henrietta Lacks.Henrietta died in 1951, and her cells are still alive and are used in research in many laboratories around the world (see also Rebecca Skloot's book "The Immortal Life of Henrietta Lacks").
To ensure that the cells were clearly visible in the photograph, they were stained with an immunofluorescent dye before photography. The protein connexin 43 glows red; it is one of the membrane proteins; it serves as a marker of epithelial cells. With its help, cells form contacts and adhere to each other, which is very important for epithelial cells, since they must form a protective layer that will not allow anything unnecessary to pass through. Nuclei are colored with blue dye, and microtubules, consisting of the class IIIβ tubulin protein (see Class III β-tubulin) - this is the “skeleton” of the cell (see picture of the day, “Colored Cytoskeleton”), are colored with green dye.
The retina is a structure made up of several layers of neurons and photoreceptor cells that enable our ability to see. For it to function properly, it needs support - nutrition and protection. They are provided by a special layer of cells - the retinal pigment epithelium (RPE). This is the outermost layer of the retina, its cells are located between the photoreceptors and the choroid of the eye. If the functioning of the RPE is disrupted, the functioning of the retina is also disrupted, up to complete loss of vision. One of the most common diagnoses of RPE dysfunction is age-related macular degeneration. To study the causes of the development of retinal diseases and develop methods for their treatment, it is precisely cell cultures of the pigment epithelium that are needed - experiments should not be carried out on a living eye!
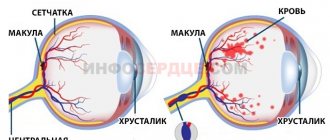
The structure of the retina. It can be seen that the pigment epithelium is the outermost layer, immediately after the sclera. Drawing from the site tvoiglazki.ru
Pigment epithelial cells contain melanin pigments (black granules inside the cells are visible under a microscope). Melanin granules absorb light that enters the eye and is not absorbed by photoreceptors, making the visible image sharper and more contrasty. In bright light, the granules migrate closer to the photoreceptors, as if enveloping them. This is necessary in order to absorb excess scattered light and make the visible image clearer. In the dark, they sink to the bottom of the cell (closer to the choroid). On the surface, pigment epithelial cells have projections that encircle the lower parts of the photoreceptors. By binding to them, the RPE performs the function of a blood-retinal barrier, which selectively allows nutrients from the blood to reach the photoreceptors and removes breakdown products into the blood. In addition, pigment epithelial cells phagocytose (that is, bite off and digest) the outer, spent parts of the photoreceptors and restore visual pigment from them in order to put it back into action.
In the body, the RPE forms a dense layer, where each cell takes the shape of a hexagon - this shape allows you to fit the maximum number of objects into a minimum area (remember a honeycomb). In laboratory conditions, cells can move more freely and take on different shapes - as long as their concentration does not become too high.
Electron micrograph of retinal pigment epithelium in a rabbit eye (not in culture). In the human eye the picture is approximately the same. On the surface of the cells, outgrowths are visible with which they wrap around the photoreceptors. Scale bar length: 20 µm. Photo from an article by XY Fang et. al., 2001. Effect of Ca(2+)-free and Mg(2+)-free BSS Plus solution on the retinal pigment epithelium and retina in rabbits
Photo © Elena Shafei, Institute of Developmental Biology named after N.K. Koltsov RAS. The material was prepared together with the “Beautiful Science” community.
On the structure of the eye, see also:
Iris (picture of the day).
Elena Shafei
Anatomy and histology
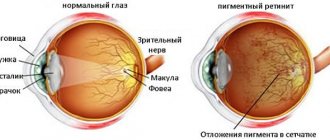
There are three parts in the Retina: the iris (pars iridica retinae), adjacent to the posterior stroma of the iris (see), the ciliary (pars ciliaris retinae), lining the inner surface of the ciliary body (see), and the optic (pars optica retinae) - the main part C. The latter is located between the optic disc and the dentate line, and in these two zones it is most firmly attached. In accordance with the structure and function of the visual part of the eye, two sections are distinguished: central and peripheral. The central section is represented by the macular zone, or yellow spot (spot, T., macula), located on the temporal side of the optic nerve head (see). The name "macula macula" refers to the orange-yellow color of this area in the enucleated eye.
The macular zone has an oval shape with a horizontal meridian of approx. 5.5 mm, in its center there is a depression - the central fossa (fovea centralis).
S.'s thickness in the area of the central fossa is 0.2–0.25 mm. At the bottom of the central fossa there is a depression with a diameter of approx. 0.2 mm - dimple (foveola); here S. has the smallest thickness - 0.08-0.1 mm. The greatest thickness of S. (0.5 mm) is noted in the perifoveal region. Towards the dentate line it decreases to 0.1 mm. Changes in the thickness of the fossa are due to the fact that the fovea contains only cones, and the central fovea is limited by the nuclei of ganglion cells lying in 5-6 rows.
The pigment epithelium of S. is tightly connected to the vitreous lamina, or Bruch's membrane (basal lamina, or basal complex, T.), the choroid proper. In front of the dentate line, the pigment epithelium of the ciliary body passes into the pigment epithelium of the ciliary body, and the remaining layers of the ciliary body into the non-pigmented epithelium of the ciliary body.
The retina has vertical and horizontal associative connections. Vertical connections are represented by a reflex arc, in which the first neuron is neuroepithelial cells - rods and cones, the second - bipolar cells, the third - ganglion cells, the axons of which form the optic nerve, chiasm (optic chiasm, T.), optic tracts (see. Visual centers, paths) and end in the external geniculate body, where the bodies of the fourth neurons are located. Horizontal connections are made through amacrine and horizontal cells, which are closely associated with bipolar cells and group them into associative fields.
Schematic representation of the microscopic structure of the retina (the layers of the retina are shown on the left, the relationship of cellular elements on the right): I - pigment epithelium; II - layer of rods and cones; III - external limiting membrane; IV - outer nuclear layer; V - outer plexiform layer; VI - inner nuclear layer; VII - internal plexiform layer; VIII - layer of ganglion cells; IX - layer of nerve fibers; X - internal limiting membrane; 1 - amacrine cell; 2 — horizontal cell; 3 - Müller cell; 4 - cores of rods; 5 - cone nucleus; 6 - cilia of pigment epithelial cells.
Microscopically, 10 layers are distinguished in S. (Fig.): 1) pigment epithelium; 2) layer of rods and cones (neuroepithelial, or photoseed layer); 3) external boundary membrane (external boundary layer, Babukhin’s membrane); 4) outer nuclear layer; 5) outer plexiform layer (outer mesh layer); 6) inner nuclear layer; 7) internal plexiform (internal mesh) layer; layer of ganglion cells (ganglionic layer); 9) layer of nerve fibers; 10) internal boundary membrane (internal boundary layer).
2) layer of rods and cones (neuroepithelial, or photoseed layer); 3) external boundary membrane (external boundary layer, Babukhin’s membrane); 4) outer nuclear layer; 5) outer plexiform layer (outer mesh layer); 6) inner nuclear layer; 7) internal plexiform (internal mesh) layer; layer of ganglion cells (ganglionic layer); 9) layer of nerve fibers; 10) internal boundary membrane (internal boundary layer).
The pigment epithelium performs the function of light absorption, phagocytosis, production of acidic mucopolysaccharides, accumulation of vitamin A; together with the vitreous plate of the choroid itself, it forms the external structures of the hematoretinal barrier. The histological structures of the pigment epithelium are adapted to its functions. Intensely pigmented hexagonal cells form a monolayer of tightly interconnected elements. The connection of their basal surfaces with the vitreous plate is carried out due to numerous folds of the cell membrane, and the lateral surfaces of the pigment epithelial cells are interconnected by the folds they form, intersecting each other, desmosomes and dense membrane complexes. These intercellular contacts prevent the passage of large-molecular proteins from the choroid proper into the C. The apical surfaces of pigment epithelial cells, facing the rods and cones, have numerous short (3 μm) and long (5-7 μm) cilia. The former are located between the terminal sections of the rods and cones, the latter - between the photoreceptors. The space between the cilia of the pigment epithelium and the outer segments of the photoreceptors is filled with glycosaminoglycans. The cytoplasm of pigment epithelial cells is polarly oriented - the basal parts of the cells contain nuclei with two nucleoli and diffuse chromatin, mitochondria, a well-defined granular endoplasmic reticulum - granular endoplasmic reticulum (see Endoplasmic reticulum), free ribosomes, Golgi complex (see Golgi complex), structures of the smooth endoplasmic reticulum (non-granular endoplasmic reticulum) and lysosomes (see). In the middle and apical part of the cells, pigment granules consisting of melanin predominate; granules have a cigar shape, length up to 1 micron, dia. 2-3 microns. In the macular zone, pigment epithelial cells are cylindrical in shape and contain a large number of pigment granules. At the periphery, the cells become flattened. Pigment epithelial cells also contain lipofuscin (see), which has the appearance of residual bodies developing from lysosomes. In addition, the cells have membrane structures associated with the function of phagocytosis of the outer sections of rods and cones, as well as microperoxisomes that take part in lipid metabolism.
The layer of rods and cones is represented by outer and inner segments (segments) of photoreceptor cells. The segments are connected to each other by a thin bridge (cilium). Rods are longer and thinner, cones are wider and shorter. In the outer segments of the photoreceptors there are discs that have a membrane structure and contain light-receiving visual pigment (rhodopsin - in rods, iodopsin - in cones). The distance between the disks in the outer segments of cones is wider than in the outer segments of rods. The internal segments of the photoreceptors are shorter, ellipsoidal in shape and contain a large number of mitochondria (especially in cones), ribosomes, glycogen granules, the Golgi complex and neural tubules. The internal segment is separated from the neighboring one by processes of Müller cells.
The external limiting membrane is a fenestrated formation formed by the processes of Müller cells. It is located at the level of the border between the internal segment of the photoreceptors and their basal part.
The outer nuclear layer is composed of the internal sections of photoreceptors. In relation to the membranes of the eye, the nuclei of the rods lie inward, the nuclei of the cones lie outward, the sizes of the latter are somewhat larger. The cytoplasm of cells contains ribosomes and a large number of nerve tubes, which pass into synaptic extension.
The outer plexiform layer is the transition synaptic zone between the first and second neurons. Extended parts of photoreceptor axons containing synaptic vesicles contact the processes of bipolar and horizontal cells. The synaptic zone forms the median line of the S., the edges are the border of two pools of blood supply; the layers lying outward from it are nourished by the choriocapillary layer (choroidal-capillary plate), the internal ones by the central artery of the retina.
The inner nuclear layer contains the nuclei of horizontal amacrine, Müllerian and bipolar cells, the latter making up the bulk. Horizontal and amacrine cells have many processes, which contact other neurons, carrying out integrating associative connections. Bipolar cells, which are the second neurons, have synaptic contacts with photoreceptors and ganglion cells. Among bipolar cells, poly- and monosynaptic cells are distinguished, the dendrites of which are located in the outer plexiform layer, axons - in the inner. The cytoplasm of bipolar cells contains mitochondria, ribosomes, and structures of smooth and granular endoplasmic reticulum. Müller cells belong to the glial elements that perform skeletal (supporting) as well as trophic functions in S. The cytoplasm of Müller cells appears denser due to the large number of microfibers.
The inner plexiform layer is the synaptic zone of the second and third neurons, as well as amacrine cells. This layer contains the capillary network C. Amacrine, bipolar and ganglion cells have synaptic connections of various structures, which is revealed by electron microscopic examination.
The layer of ganglion cells, which are the third neuron, has an uneven thickness; in the nasal half of the S. the cell bodies are located in one row, in the temporal half - in two, the cell density decreases towards the periphery of the S. The cells have poly- and monosynaptic connections with bipolar and amacrine cells, the axons of which lie in the inner plexiform layer. The axons of ganglion cells form a layer of nerve fibers. The shape of the cells is round, the cytoplasm is dense, fibrous, contains aggregated structures of granular endoplasmic reticulum (Nissl substance), mitochondria, free ribosomes and a large number of neural tubes, especially in dendrites and axons. Lipofuscin granules are found in older people.
The layer of nerve fibers consists of axons of ganglion cells, which form the optic disc (see). From the macular zone, nerve fibers go directly to the temporal half of the optic nerve head, forming the so-called. papillomacular bundle.
The internal limiting membrane, formed by Müllerian fibers, separates the retina from the vitreous limiting membrane.
Blood supply
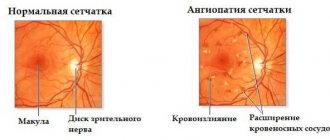
. The anatomical and physiological features of the eye include a double blood supply: due to the vessels of the choroid proper and the central artery of the eye. Depending on the source of nutrition, the eye is divided into external and internal parts; the boundary between them is the synaptic zone of the outer plexiform layer. The outer part of the eye, which includes a layer of rods and cones (the first neuron), is supplied with blood by the vessels of the choroid proper (choriocapillary layer). The capillaries of this layer are fenestrated, which determines the free release of large-molecular protein into the intercellular spaces of the choroid proper of the eyeball. The central artery of the S., which supplies blood to the inner part of the S., where the second and third neurons are located, is dichotomously divided into four main branches that supply the four quadrants of the S. Veins run parallel to the arteries. Near the optic disc, the central artery has a diameter of 0.1 mm and a wall thickness of 18 μm. In the same area, 5-7 layers of pericytes are distinguished in the wall of the artery, 2-3 in its equatorial branches, and 1-2 layers on the periphery of the artery. The endothelial cells of blood vessels are oriented circularly or obliquely relative to the axis of the vessel. Over the age of 50 years, there is a decrease in the number of pericytes and endothelial cells. Along the periphery, S.'s vessels are surrounded by perivascular glia, consisting of astrocytes. S.'s capillary network is located between the feeding artery and the draining vein.
Fundus abnormalities
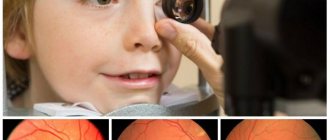
The size, position and shape of the optic disc varies greatly. There are vascular anomalies of the optic nerve head and retina, choroidal and optic nerve colobomas, and pigment hyperplasia on the retina. Optic disc anomalies include megalopapilla, disc hypoplasia, oblique disc exit, disc coloboma, optic pit, optic disc drusen, myelin fibers, vascular abnormalities, persistent hyaloid system, and morning glow sign.
Enlargement of the optic nerve head - megalopapilla - is more often observed with myopic refraction. Ophthalmoscopically, a pale, enlarged optic disc is detected. The pallor of the disc in these cases is due to the distribution of axons over a larger area and better visibility of the lamina cribrosa.
Reduction of the optic disc - hypoplasia (Fig. 3-1) - is more common in patients with hypermetropia. In this case, the size of the disc is small in relation to the retinal vessels. Often in these cases there is slight tortuosity of the retinal vessels. The optic disc is surrounded by a chorioretinal or pigment ring.
The oblique exit of the optic disc (Fig. 3-2, 3-3) can be unilateral or bilateral. Refraction in these patients is often defined as myopic astigmatism. The optic disc is of an unusual shape with prominence of one edge, which creates the impression of blurred boundaries. Retinal vessels often have an unusual course, spreading towards the nasal side. Oblique exit of the optic nerve head can be combined with thinning of the macula, detachment of the pigment epithelium or neuroepithelium.
Coloboma of the optic nerve head includes an extensive defect of the disc and peripapillary zone, and is often combined with coloboma of the choroid. In this case, visual functions are sharply reduced, defects are determined in the field of vision, corresponding in localization to coloboma (Fig. 3-4, 3-5).
The optic disc pit is a mild form of coloboma.
In some cases, pigmentation of the optic nerve is observed, when pigment is deposited on the surface of an unchanged disc in the form of stripes or spots that pass onto the disc from the peripapillary zone.
Myelin fibers are found in one or both eyes and ophthalmoscopically have a banded appearance and a whitish-yellowish color. Myelin fibers are most often localized in the peripapillary zone or on the optic nerve head, but can also be located on the periphery of the fundus. Visual functions are not affected (Fig. 3-6, 3-7, 3-8).
The persistent hyaloid system consists of papillary and prepapillary membranes, which can take the form of a massive connective tissue film or thin cords extending from the optic disc into the vitreous body. Changes are usually one-sided. Visual acuity with small changes remains high, but with extensive coarse connective tissue membranes it sharply decreases down to hundredths (Fig. 3-9).
The “morning glow” symptom is ophthalmoscopically characterized by a mushroom-shaped prominence of the optic disc surrounded by a raised chorioretinal pigmented ring. The vessels on the disc have abnormal division and course. Visual functions do not change (Fig. 3-10).
Drusen of the optic disc and optic disc fossa, as the most common anomalies, are described as separate nosological entities in the chapter “Pathology of the optic disc”.
Vascular anomalies on the optic nerve head can be observed in the form of vascular loops and pathological tortuosity. Visual functions are not affected, but vascular changes can subsequently lead to impaired microcirculation and thrombus formation (Fig. 3-11).
Among the anomalies of the fundus, there are also colobomas of the choroid, underdevelopment of the macular region, which are often combined with other developmental anomalies (aniridia, microphthalmos). They can be a true developmental anomaly or develop as a result of fetal diseases, especially toxoplasmosis. Histological studies have shown that the retina in the area of choroidal coloboma is preserved, although greatly reduced, the pigment epithelium is often absent, the choroid is underdeveloped, and the sclera is thinned. Colobomas of the choroid that do not affect the central zone of the fundus do not reduce visual acuity and usually become a finding of the ophthalmologist (Fig. 3-12 3-12a 3-12b).
Congenital accumulations of pigment are often multiple, have the form of spots and are grouped in separate sectors of the fundus; they do not cause a decrease in visual acuity or changes in the visual field (Fig. 3-13, 3-14).
Physiology
In S., a complex process of converting light energy into a nerve impulse occurs. The function of absorbing light from the visible part of the solar spectrum (approximately from 390 to 760 nm) and transmitting the energy thus obtained by the photoreceptor is performed by visual pigments, which are contained in both rods and cones. Visual pigments are complex colored proteins (chromoproteins). That part of them, which absorbs light, is called a chromophore. In chem. In relation to this, it is an aldehyde of vitamin A (retinal). The protein of visual pigments, with which retinal is associated, is called opsin (see Visual pigments).
Under the influence of light, a photochemical reaction occurs in the visual pigment of rods - rhodopsin, or visual purple, which is externally manifested by its fading. When rhodopsin (see) decomposes, opsin and transretinal are formed, which, under the action of a special enzyme, turns into retinol (see). The latter then migrates from the photoreceptor to the pigment epithelium, with the cilia of which the photoreceptor is in close contact. Rhodopsin resynthesis occurs in the dark. Vitamin A passes back from the pigment epithelium to the receptor and, oxidizing, turns into a special aldehyde form 11-cisretinal. Only this form is capable of re-forming rhodopsin when combined with opsin. The obligatory participation of vitamin A in the dark adaptation of the eye explains the disorder of twilight vision due to insufficiency of this vitamin in the body (see Hemeralopia).
Much less is known about the photochemical process, which occurs under the influence of light in cones. Instead of rhodopsin, they contain another visual pigment - iodopsin. The process of decomposition of the latter requires significantly higher light intensity than the process of decomposition of rhodopsin. In the dark, the decayed pigment of the cones is restored again without any participation of the pigment epithelium, and at a much faster rate than rhodopsin. The heterogeneity of iodopsin is associated with the existence in S. of three different elements (or components) of cones, each of which is intended for the perception of only one of the three primary colors - red, green and blue. When color rays act on the eye, all three elements are excited, but to varying degrees, which allows us to perceive the whole variety of color shades.
Physiol. The substrate for excitation of photoreceptors under the influence of light is the electrical potential, which is generated by one of the intermediate products of the transformation of visual pigment. There are two types of receptor potentials: early, with virtually no latent period, and late, with a latent period of more than 1.5 ms. From cones and rods, nervous excitation is transmitted to bipolar cells, and from them to ganglion cells. The code for the intensity of the signal sent to the brain along the axons of ganglion cells—the fibers of the optic nerve—is the frequency of the pulse discharges.
The functional unit of the brain is the receptive field. It is a collection of rods and cones associated with a single ganglion cell. Some receptive fields respond only to the light being turned on, others only to the light being turned off, and others to both the light being turned on and off. Ganglion cells have been found in animals that selectively respond only to certain characteristics of a stimulus, for example, to the movement of a light (or dark) spot or strip in only one specific direction. Such neurons are called detectors. Receptive fields are not constant. Depending on the changing conditions and tasks of visual perception, their functional restructuring occurs.
At the level of the Retina, due to the spatio-temporal summation of the light stimulus, inhibitory interaction between receptive fields and between zones within the fields themselves, the contours of the image are emphasized. The information of the chapter is transmitted to the overlying sections of the visual analyzer (see). arr. about those parts of the image where there is a difference, gradation of brightness and contains the greatest novelty and information content.
The minimum amount of light energy that causes the sensation of light characterizes the absolute light sensitivity of the eye. By changing its visual system, it adapts to different brightness levels over a wide range - from 10-6 to 104 nits (cd/m2). Light sensitivity increases sharply in the dark (dark adaptation) and decreases when moving from less illumination to greater illumination (light adaptation). If there are areas in the field of view with unequal brightness, their difference is assessed through the contrast, or discriminative, sensitivity of the eye, which makes it possible to distinguish the spatial configuration of images (see Light perception).
According to the generally accepted theory of duality of vision, two of its apparatuses are distinguished - central (or cone) and peripheral (or rod). The first is a daytime vision apparatus, which provides color perception and detailed distinction of objects in the surrounding world, the second is a twilight vision apparatus, which is sensitive to very weak light, but does not perceive color shades (see Vision).
Research methods
The main methods for studying the functional ability of the retina are the determination of visual acuity (see) and perimetry (see). The first method makes it possible to judge the state of central vision, the second - about peripheral vision, i.e., about the boundaries of the visual field and the presence of defects in it - scotoma (see Scotoma). A more detailed description of the latter can be obtained using campimetry (see). To study the function of color vision, special tables or a device are used - anomaloscope (see Color vision). Determination of the value of light sensitivity and the course of its change under conditions of adaptation of the eye to darkness is carried out using special devices - adaptometers (see Visual adaptation). For an objective assessment of S.'s condition, ophthalmoscopy is used (see). Normal S., when examined in achromatic light, almost does not reflect rays and therefore remains transparent and invisible. Only the slightly darker color of the macular zone and the light reflex along its edge are clearly visible. For a more thorough examination of S., they resort to ophthalmoscopy in red-free light, during which predominantly blue-green rays penetrate into the eye, which, reflected from S., make it clearly visible (see Fundus).
The diameter of the retinal vessels is determined by ophthalmic calibrometry, projecting various measuring systems onto the fundus of the eye during ophthalmoscopy.
To assess the physiological state of the retina, electroretinography (see) and a study of the electrical sensitivity of the eye are used (see Electrophysiology of the organ of vision). The electroretinogram reflects the state of the outer layers of S., the electrical sensitivity - its inner layers.
A valuable method for studying the hemodynamics of the eye is fluorescence. angiography (see) - photographing the vessels of the choroid and S. after they are contrasted with fluorscein.
Pathology
Pathological changes in the Retina manifest themselves in the form of diffuse and limited opacities, hemorrhages and pigmentations (see Retinitis, Retinopathy).
Diffuse opacities of large or small sizes give S. a dull gray color and are especially pronounced in the area of the optic nerve head. Limited foci of S.'s turbidity can have different shapes, sizes, and colors—white, light yellow, or bluish-yellow. Located in the layer of nerve fibers, they take on a streak-like shape; in the macular zone they form a figure resembling a star. The round shape and pigmentation of the lesions are observed when the process is localized in the outer layers of the C. As a result of the subsequent atrophy of the choroid, the sclera is exposed in these areas and they take on the appearance of white lesions, often surrounded by a pigmented rim.
Fresh hemorrhages in S. are cherry-red in color and vary in size. When localized in the layer of nerve fibers, they appear in the form of radial strokes or triangles with their apex facing the optic nerve head. Preretinal hemorrhages are round or transverse oval in shape.
The main forms of S.'s pathology are malformations of its development, damage, diseases of a neurocirculatory nature (retinopathy), inflammatory diseases of the S. (retinitis), its dystrophic changes (or degeneration) and neoplasms of the S. Diseases of the S. are manifested by a decrease in central and peripheral vision, impairment color vision, dark adaptation and loss of vision. Their character and severity depend on the localization and prevalence of patol. process.
Abnormalities of retinal development are rare. These include the congenital fold of the S., which cannot be treated, as well as macular coloboma (see Coloboma) in the form of a light or yellowish pigmented focus with sharp boundaries.
Damage to the retina most often manifests itself in the form of contusional edema (see Retinopathy) and retinal detachment (see), hemorrhages into its layers, possible ruptures of the retina and its separation from the dentate line (see Eye, damage).
Disorders of blood circulation in the retina occupy a large place in its pathology. If the central artery of the S. is obstructed due to spasm, thrombosis and embolism, partial or complete loss of vision suddenly occurs. The fundus of the eye becomes milky white due to swelling C. Against this background, the macular zone is highlighted in dark red (the “cherry pit” symptom). The arteries are sharply narrowed.
With thrombosis of the central vein of S., swelling of the optic disc is noted in the fundus (see) (see Congestive nipple). The veins on the surface of the disc and around it are dilated, tortuous, dark in color, and massive hemorrhages are noted along the veins.
If the patency of S.'s vessels is impaired, emergency care is required in a hospital setting. For treatment, vasodilators and antispasmodics, anticoagulants, fibrinolytic agents, lipotropic substances, vitamins are used, and dehydration therapy is carried out.
The prognosis for spasm of the central artery of the S. is favorable; with other types of obstruction, visual acuity decreases sharply. With thrombosis of the central vein of S., visual acuity does not decrease as sharply as with obstruction of the retinal artery.
Retinal periphlebitis (Eales disease) is one of the severe forms of retinal damage. It usually develops against the background of tuberculosis, blood diseases, viral infections, etc. There are four stages of periphlebitis. In the first stage, dilation, tortuosity, and intermittency of the C veins are noted. In the second stage, periphlebitis forms and retinal hemorrhages appear. In the third stage, recurrent hemorrhages into the vitreous are observed, in the fourth stage - gliosis, detachment of the vitreous. Treatment begins with the treatment of common predisposing diseases. Corticosteroids, hyposensitizing agents are also used (see Hyposesensitization), photocoagulation of the affected veins C. The prognosis for vision is unfavorable.
Dystrophic changes in the retina (retino-dystrophy). The most common and severe form of this pathology is retinal pigmentary degeneration (see Tapetoretinal dystrophies).
Senile retinal degeneration usually occurs in people over 60 years of age. Its etiology and pathogenesis have not been sufficiently studied. Manifests itself in the form of accumulations of pigment in the area of the macula; then dotted merging patches of yellow color appear here and this entire area gradually acquires a dark red or yellowish-brown color.
One of the forms of senile degeneration of S. is Kunt-Junius disease, which is manifested by mottling of the macula area, and then the appearance of a disc-shaped edematous focus here, followed by new formation of connective tissue.
Cyst-like degeneration of S. is characterized by the appearance of small thinned areas in the form of honeycombs in the area of the macula.
For the treatment of S.'s dystrophy, vasodilators, vitamins, tissue therapy (see), anabolic steroid drugs, ultrasound therapy, and the method of neovascularization of S. are used.
Treatment started in the early stages of S.'s dystrophy, in some cases helps to stabilize the process.
Retinitis occurs due to the introduction of virulent microflora into S., under the influence of light or ionizing radiation, or with damage to the retina (see Retinitis).
Retinopathy can be either an independent disease or a manifestation of other diseases accompanied by circulatory and metabolic disorders in the S. One of the forms of retinopathy is the Berlin cloudiness of the S., which occurs with contusions of the eyeball and manifests itself in the form of a whitish cloudiness, usually along the periphery of the S. ( see Retinopathy).
Tumors - see Retinoblastoma.
Symptoms of the disease
The symptoms of this disease are not always noticed by women. The disease is asymptomatic in some cases. Characteristic signs of the disease are:
- Unusual cervical secretion. The mucous discharge is too excessive, the woman is forced to regularly use sanitary pads to protect her underwear.
- Intermenstrual bleeding (spotting). Not the cyclical nature of menstruation, when endocervical hyperplasia occurs simultaneously with other hyperplastic processes.
Before and after menstruation
Hyperplasia is often suspected as monthly discharge changes:
- Heavy bleeding appears after a certain delay in menstruation. In such a situation, the thickness of the endometrial ball increases sharply. Menstruation has a thin consistency, with clots. Such adjustments are painful because intrauterine pressure is increased. The tissues of the uterus begin to expand, and spasms are likely due to compression of the blood vessels.
- Scanty menstruation is characteristic of uneven changes in the endometrium. The fabric becomes covered with lesions. During menstruation, these particles are not rejected. Due to the reduction of the layer, the regula are scarce.
- With hyperplasia, bleeding occurs that is not associated with menstruation. This is caused by tissue proliferation.
- Bleeding appears after sexual intercourse due to damage to the cervix. They are provoked by blood vessels that burst.
During pregnancy
Pathology during pregnancy is difficult to recognize, since cell division eliminates the symptoms of the disease. It is determined only after a certain examination. The disease can be detected when the placenta grows:
- a deviation in the child’s heartbeat appears;
- the sensation of internal movement of the developing fetus is noticeably reduced;
- with a sharp decline in heartbeat contractions, oxygen starvation of the developing fetus occurs, and its hypoxia cannot be ruled out.
When a pregnant woman is diagnosed with diabetes, the disease manifests itself as polyhydramnios. Probably vaginal burning, an increase in the amount of mucus secreted from the genitals.
Operations
Surgical interventions on the retina are performed mainly for its detachment (see Retinal detachment) and ruptures. Laser microsurgery methods are used for retinopathy, for example. diabetic, hypertensive (see Laser, lasers in ophthalmology).
Bibliography:
Arkhangelsky V. N. Morphological foundations of ophthalmoscopic diagnosis, M., 1960; Kovalevsky E.I. Children's ophthalmology, M., 1970; aka Eye Diseases” M., 1980; Krasnov M. L. Elements of anatomy in the clinical practice of an ophthalmologist, M., 1952; Lopashov G.V. and Stroeva O.G. Development of the eye in the light of experimental research, M., 1963; Multi-volume guide to eye diseases, ed. V. N. Arkhangelsky, vol. 3, book. 1, p. 11, M., 1962; Stroeva O. G. Morphogenesis and congenital anomalies of the eye of mammals, M., 1971; Tamar G. Fundamentals of sensory physiology, trans. from English, M., 1976; Physiology of sensory systems, part 1 - Physiology of vision, ed. G.V. Gershuni, p. 88, 126, L., 1971; Etingof R. N. Retinal rhodopsin, position and diffusion in the membrane, nature, Usp. modern biol., t. 84, v. 4, p. 53, 1977, bibliogr.; Hogan M. J., Alvarado J. A. a. Weddell J. E. Histology of the human eye, Philadelphia, 1971; Kuwabara T. a. Сogan DG Studies of retinal vascular patterns, Arch. Ophthal., v. 64, p. 904, 1960; Lehrbuch und Atlas der Augenheilkunde, hrsg. v. H. Pau, S. 369, Stuttgart - NY, 1980; The retinal pigment epithelium, ed. by K. M. Zinn a. M. E. Marmor, Cambridge - L., 1979; System of ophthalmology, ed. by S. Duke-Elder, v. 10, St Louis, 1967.
E. S. Avetisov; G. G. Ziangirova (an., GIST., embr.).